
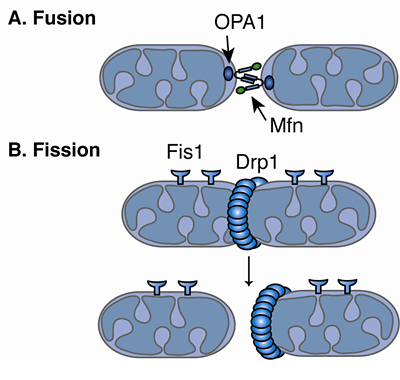
Recent papers have also revealed interplay between fusion and fission in most cases, they appear to be sequential, cycle-forming events ( Twig et al., 2008 Wang, 2012). The outer membrane protein Fis1 and the cytoplasmic protein Drp1 are responsible for fission events (for a review, see Knott et al., 2008). The key molecules responsible for fusion are Mitofusin-1 and -2 (Mfn1 and 2, respectively), which are located in the outer mitochondrial membrane, and OPA1, which is located in the inner mitochondrial membrane. The molecular mechanisms underlying mitochondrial fusion and fission events are relatively well-known. Perturbations of the mitochondrial fusion–fission cycle cause autosomal dominant optic atrophy and Charcot–Marie–Tooth type 2A and appear to be involved in the pathogenesis of several neurodegenerative diseases ( Westermann, 2010). Failure of mitochondrial fusion–fission dynamics has been linked to several diseases. Mitochondrial fission contributes to quality control by favouring removal of damaged mitochondria via mitophagy ( Gomes et al., 2011) and may facilitate apoptosis in conditions of cellular stress ( Suen et al., 2008). Beyond the control of morphology, the mitochondrial fusion–fission cycle appears to be also critical in regulating cell death and mitophagy. The latter is particularly important in neurons, which have a unique bioenergetic profile due to their dependence upon energy from mitochondria and their specialised, compartmentalised energy needs. The balance of these two processes determines organelle shape, size and number and is critical for organelle distribution and bioenergetics. This knowledge will increase understanding of the dynamic mitochondrial functions in cells and in particular, the pathogenesis of mitochondrial-related neurodegenerative diseases. Taken together, our results provide a novel insight into the complex crosstalk between different processes involved in mitochondrial dynamics. Our main findings demonstrate that: (1) the probability of a single mitochondrion splitting is determined by its length (2) the probability of a single mitochondrion fusing is determined primarily by its motility (3) the fusion and fission cycle is driven by changes in mitochondrial length and deviations from this cycle serves as a corrective mechanism to avoid extreme mitochondrial length (4) impaired mitochondrial motility in neurons overexpressing 120Q Htt or Tau suppresses mitochondrial fusion and leads to mitochondrial shortening whereas stimulation of mitochondrial motility by overexpressing Miro-1 restores mitochondrial fusion rates and sizes. Here, we took advantage of modern visualisation techniques to provide a clear explanation of how fusion and fission correlate with mitochondrial length and motility in neurons. However, an integrated and quantitative understanding of how fusion–fission dynamics control mitochondrial morphology has not yet been described. These dynamics control mitochondrial morphology, which in turn influences several important mitochondrial properties including mitochondrial bioenergetics and quality control, and they appear to be affected in several neurodegenerative diseases. Mitochondrial fusion–fission dynamics play a crucial role in many important cell processes.


 0 kommentar(er)
0 kommentar(er)
