
In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. What is an orbital diagram?Īnother way to represent an electron configuration is through an orbital diagram. Using the short-hand method, we will place neon in brackets then continue the electron configuration. The last noble gas that was passed for chlorine was neon (Ne). ChlorineĬhlorine is located in period three of the p-block. Using the short-hand method, we will place argon in brackets like this and then continue the electron configuration after argon. The last noble gas that was passed for bromine was argon (Ar). Remember, for electron configurations you work left to right and down the periods until you get to the element you’re focusing on. Bromineīromine is located in period four of the p-block. The shorthand method uses the group 18 elements, the noble gases, as a bookmark. You can still write out every single subshell if you would like, but to save time it is good to know the shorthand method of electron configurations. Writing Electron Configurations – Shorthand Method The electron shell configuration for phosphorous would be 1s 22s 22p 63s 23p 3. It is located in the third period and within the p-block. Now, let’s find phosphorus on the periodic table. If we count the electrons in each orbital for Carbon’s configuration, we get 2+2+2= 6! Phosphorus We can also reaffirm this answer by noticing that carbon is number six on the periodic table, and therefore has six electrons. The electron shell configuration is 1s 22s 22p 2. Carbon is located in the second period and in the p-block, so it’s highest energy electrons will occupy the 2p orbital. Carbonįollow the periodic table starting at hydrogen just as the example for Lithium. Only the electron occupancy changes, which we denote by changing the superscript from 1 to 2. The electron configuration for Lithium is therefore 1s 2 2s 1.Ī Note: The first half of lithium’s electron configuration is written using only “1s 2” and not “1s 11s 2” because between Hydrogen and Helium, the energy level and orbital do not change. We start with our attention on hydrogen (1s 1), move to Helium (1s 2), and then to Lithium (2s 1). One again we will use the example of Lithium. At each preceding element, pay attention to the energy level and block it represents. To find the electron configuration of an element, start at hydrogen and trace across each period until your target element is reached. Writing Electron Configurations – Examples Groups or blocks of the periodic table share the same sublevel, and are divided as seen in the following diagram. Sublevels are indicated by letters s, p, d, and f. Each successive integer generally represents a higher energy level than the last. The principle energy level is indicated by an integer (1, 2, 3, …7) that corresponds with the periods on the periodic table. The periodic table is a helpful tool in writing these configurations.

Lithium, containing three electrons, has two electrons occupying an s orbital at the first energy level, and one electron occupying an s orbital at the second energy level. The electron configuration for Lithium is: 1s 2 2s 1 Systems with a greater number of electrons will occupy a greater amount of energy levels. In this case, there are two electrons in an s orbital with the principle energy level of one. The first integer, 1, gives us the principle energy level, the letter s represents the type of orbital (sublevel), and the superscript 2 gives us the electron occupancy. Here is the electron configuration for Helium: 1 s 2 Writing Electron ConfigurationsĮlectron configurations have a standard notation that tells you the principle energy levels and sublevels that electrons occupy. Electron configurations are represented by standard written notation, or by using orbital diagrams. Systems with a greater number of electrons will occupy a greater amount of energy levels, meaning that they also will utilize higher energy levels. Electrons occupy orbitals that have characteristic levels of energy. The electron configuration is a description of where electrons are in a molecule or atom. If you enjoy this tutorial, be sure to check out our others linked below! Related Articles

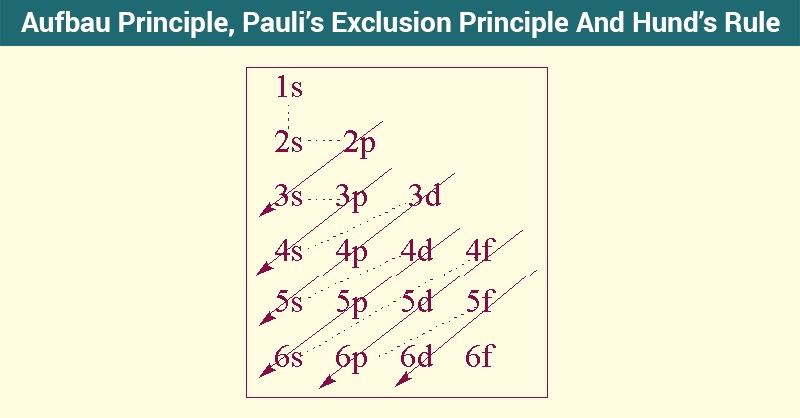
You will learn Aufbau’s principle, Hund’s rule and the Pauli exclusion principle.
#Pauli exclusion principle worksheet how to#
In this tutorial, you will learn how to find and write the electron configurations and orbital diagrams for various elements using the periodic table.


 0 kommentar(er)
0 kommentar(er)
